Experimental design decisions#
A number of critical decisions must be made when designing a quantitative bioimaging experiment. Many of these decisions will be deeply dependent on what is possible given the biology you wish to study, for example:
You will always prefer live imaging to fixed imaging if it is important to assess the dynamics of a given process
You may need to perform special processes such as tissue clearing if it is important to image deep into a relatively opaque specimen
You cannot rely on genetic tagging with fluorescent proteins if your model system is not genetically tractable to such manipulations
You have to image at a particularly high resolution to confidently assess interactions between two molecules imaged in the same system
It is therefore extremely important to think through all of the aspects of your biological question before ever picking up a pipette or a slide. Many sample preparation decisions are deeply entwined with the availability and suitability of particular microscopes; see that section for more information.
Mounting#
Glass coverslips#
Many imaging applications involve mounting on glass coverslips, either directly or using a coverslip mounted into a dish. While there is a wide range of coverslip sizes and shapes, the most important attribute is the coverslip thickness. Coverslip grade dictates the expected thickness and tolerance. These factors are important because most microscope manufacturers assume a specific coverslip thickness (0.17mm) in the design of objective lenses to minimize aberrations. These aberrations tend to affect the brightness and axial resolution, reducing signal to noise ratio, sharpness, and resolution. The tolerance of the coverslip (to minimize the variability in thickness) is essential for super-resolution techniques or intensity measurements in images collected with high numerical aperture objectives. Other applications do not require the mounting of samples onto glass or plastic but instead have the sample and the objective lens immersed in the same medium.
Grade |
Nominal thickness [mm] |
Thickness range [mm] |
|---|---|---|
#1.5 |
0.17 |
0.16 - 0.19 |
#1.5H |
0.17 |
0.165 - 0.175 |
Mounting media#
The refractive index the sample is placed in, as well as the refractive index of the glass and the medium between the objective and the sample are all critical to determining the achievable resolution. The mounting media can have other important optical and/or experimental properties; it is important to use the correct mounting media for experiment planned.
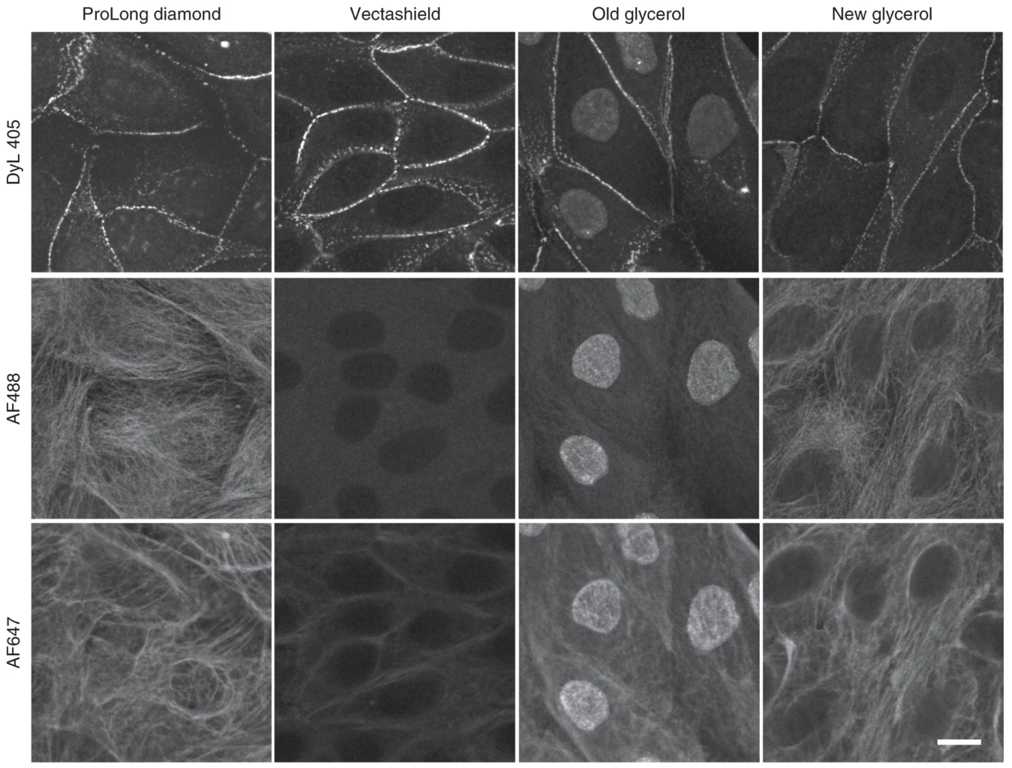
Fig. 3 Effects of mounting media on staining with various fluorophores. Adapted from Jonkman J., Brown C.M., Wright G.D et al. Tutorial: guidance for quantitative confocal microscopy. Nat Prot 15, (2020) 5#
Fluorophore selection#
Fluorophores are molecules that are able to emit light upon absorption of a photon, typically of shorter wavelength. The fluorophores relevant to biomedical research can be small molecules organic dyes (FITC, Alexa Fluor 488) that bind specific cell structure (e.g., DAPI, MitoTracker), fluorescent analogues of small molecules (e.g., phalloidin, fluorescent amino acids) or fluorescent proteins. Some of the important properties to consider when choosing fluorophores are listed below:
Excitation/emission spectra of each fluorophore
Brightness (dyes tend to be brighter than proteins)
Photostability
Propensity to oligomerize (in the case of fluorescent proteins)
Phototoxicity (when imaging in live samples)
Understanding fluorophores, microscope specifications (light source, filters, detector), and analysis goals are key in selecting the appropriate fluorophore(s) to address a scientific question. See the section on reproducibility for more information.
